Celebrity News | Interesting updates about the latest celebrity news
Valence: The Essential Guide To Understanding Its Role In Chemistry
Why is "Valence: The Essential Guide To Understanding Its Role In Chemistry" important to you?
Editor's Notes: "Valence: The Essential Guide To Understanding Its Role In Chemistry" have published today, 2023-04-20. It will help you in understanding the role of valence in chemistry, how to determine valence, and how to use valence to predict chemical behavior.
After doing some analysis and digging into information, we made the Valence: The Essential Guide To Understanding Its Role In Chemistry. We put together this Valence: The Essential Guide To Understanding Its Role In Chemistry guide to help you make the right decision.
Key differences or Key takeaways:
Transition to main article topics:
FAQ
The following section provides answers to frequently asked questions about valence, an essential concept in understanding chemical reactions and bonding.

Understanding Firewall Traversal: An Essential Guide | NSC - Source netseccloud.com
Question 1: Can valence electrons be lost or gained by atoms?
Yes, valence electrons are the outermost electrons of an atom, and they can be lost or gained during chemical reactions to form ions.
Question 2: How does valence determine the reactivity of an element?
Elements with a high valence, such as halogens, tend to be more reactive as they have more valence electrons available to participate in chemical bonds.
Question 3: What is the relationship between valence and oxidation states?
The valence of an atom is related to its common oxidation states. For example, an atom with a valence of 2 can exhibit oxidation states of +2 or -2.
Question 4: Can valence change in different compounds?
Yes, the valence of an element can vary depending on the compound it forms. For instance, oxygen has a valence of 2 in water but a valence of 1 in hydrogen peroxide.
Question 5: What is the significance of valence in molecular bonding?
Valence is crucial in determining the type and strength of chemical bonds formed between atoms. It helps predict the formation of covalent, ionic, or metallic bonds.
Question 6: How does valence affect the properties of compounds?
Valence influences the physical and chemical properties of compounds, such as their solubility, melting and boiling points, and chemical reactivity.
Understanding the concept of valence is fundamental to comprehending the behavior of atoms during chemical reactions and the properties of the compounds they form.
Continue reading:
Tips on Valence from Valence: The Essential Guide To Understanding Its Role In Chemistry
Valence, the heart of understanding chemical bonding and reactions, deserves careful attention. To enhance your grasp of this fundamental concept, here are practical tips, rooted in scientific principles, to illuminate its importance in chemistry.
Tip 1: Visualize Valence Electrons as the Key Participants
Conceptualize valence electrons as the electrons residing in the outermost shell of an atom. These electrons, driven by their high energy and mobility, actively engage in chemical bonding, shaping the molecule's properties and reactivity.
Tip 2: Embrace the Octet Rule as a General Guiding Principle
Except for hydrogen and helium, the octet rule asserts that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons. Understanding this concept provides a valuable framework for predicting and comprehending chemical bonding.
Tip 3: Identify the Electron Configuration of Elements
The electron configuration of an element reveals the number of valence electrons it possesses. This crucial information enables you to understand the element's potential for forming chemical bonds and the type of bonding it prefers. For instance, elements with valence electrons in the "ns²npx" configuration tend to form covalent bonds.
Tip 4: Utilize Valence to Predict Chemical Reactivity
Valence directly influences an element's chemical reactivity. Elements with high valence are more reactive than those with low valence. For example, sodium, with one valence electron, is highly reactive, while noble gases, with a full valence shell, are chemically inert.
Tip 5: Explore the Relationship between Valence and Oxidation States
The valence of an element is closely related to its oxidation state, which reflects the number of electrons lost or gained by an atom in forming a chemical bond. Understanding this relationship deepens your comprehension of chemical bonding and redox reactions.
By incorporating these tips into your study of valence, you can effectively grasp its significance in deciphering chemical behavior and reactions.
Valence: The Essential Guide To Understanding Its Role In Chemistry
Valence, a fundamental concept in chemistry, plays a crucial role in determining the chemical properties of elements and their interactions with each other. Here are six key aspects that provide a comprehensive understanding of valence:
- Atomic Number: Determines the number of valence electrons.
- Electronegativity: Influences the ability of an atom to attract valence electrons.
- Bonding: Valence electrons participate in forming chemical bonds with other atoms.
- Oxidation State: Describes the charge of an atom based on valence electrons lost or gained.
- Electron Configuration: The arrangement of valence electrons in atomic orbitals.
- Valence Shell: The outermost electron shell where valence electrons reside.
These aspects are interconnected, providing a holistic understanding of valence. For instance, electronegativity and valence electrons determine the type of bond formed, which influences the oxidation state of the participating atoms. Understanding valence is essential for predicting chemical reactivity, molecular structure, and properties of compounds.
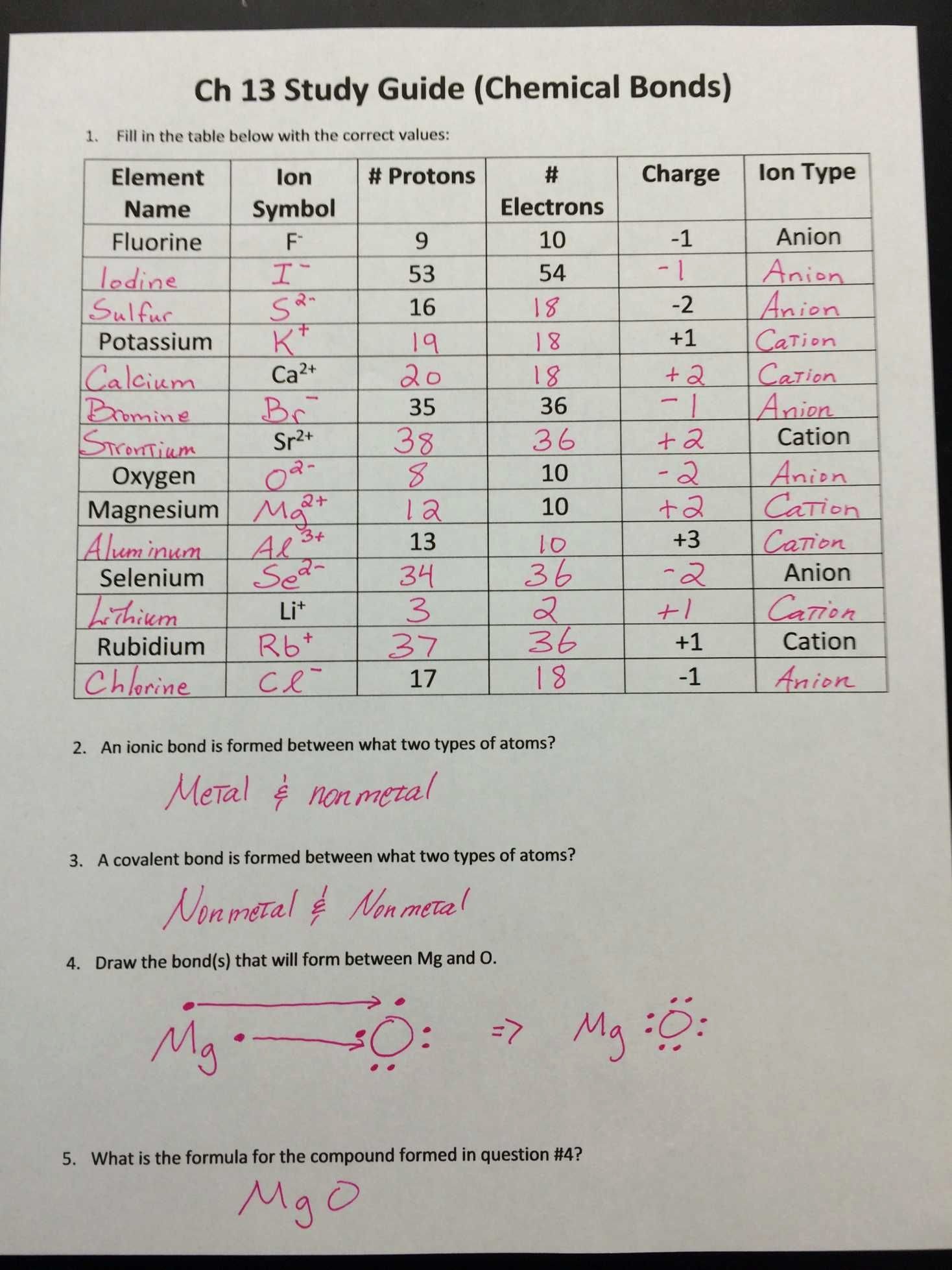
Electrons Worksheet For Grade 5 - Source lessonlibjackanapes.z22.web.core.windows.net

Carte Valence : Plan Valence - Routard.com | Sites touristiques, Guide - Source www.pinterest.fr
Valence: The Essential Guide To Understanding Its Role In Chemistry
Valence, a fundamental concept in chemistry, describes the combining power of atoms and serves as a crucial factor in determining the structure and properties of chemical compounds. Understanding valence is essential for comprehending chemical reactions and their outcomes, as it dictates the number and type of bonds that an atom can form. Valence electrons, the outermost electrons in an atom's electron configuration, play a pivotal role in chemical bonding and reactivity.

Your Handy Guide To Understanding Conditional And Absolute Discharges - Source issuu.com
Valence plays a significant role in various aspects of chemistry, including predicting chemical reactivity, determining the stability of compounds, and understanding the behavior of elements in chemical reactions. By comprehending the valence of different atoms, scientists can predict the types of bonds they will form and the overall structure of the resulting molecules. Valence also helps explain why certain elements exhibit specific properties, such as their ability to conduct electricity or form particular types of compounds.
In practical applications, understanding valence is essential in fields such as materials science, drug design, and environmental chemistry. By manipulating the valence of atoms, scientists can tailor the properties of materials for specific purposes, such as improving the efficiency of solar cells or designing new pharmaceuticals. Additionally, understanding valence helps predict the fate and transport of environmental pollutants, allowing for the development of strategies to mitigate their impact.
Conclusion
Valence, a cornerstone of chemistry, is pivotal for understanding chemical bonding, reactivity, and the properties of different elements and compounds. By delving into the concept of valence, scientists gain insights into the behavior of atoms and molecules, enabling them to predict and manipulate chemical reactions for various applications. From the design of novel materials to understanding environmental processes, valence plays a critical role in advancing our scientific knowledge and shaping technological advancements.
The exploration of valence continues to expand our understanding of the chemical world, opening avenues for further discoveries and innovations. As we delve deeper into the intricacies of valence, we unlock the potential to solve complex chemical problems and harness the power of chemistry to address global challenges.